Molecular Chaperones Assist Proteins in the Formation of
- Assist in folding. Answer 7 Which of the following is the most general term.
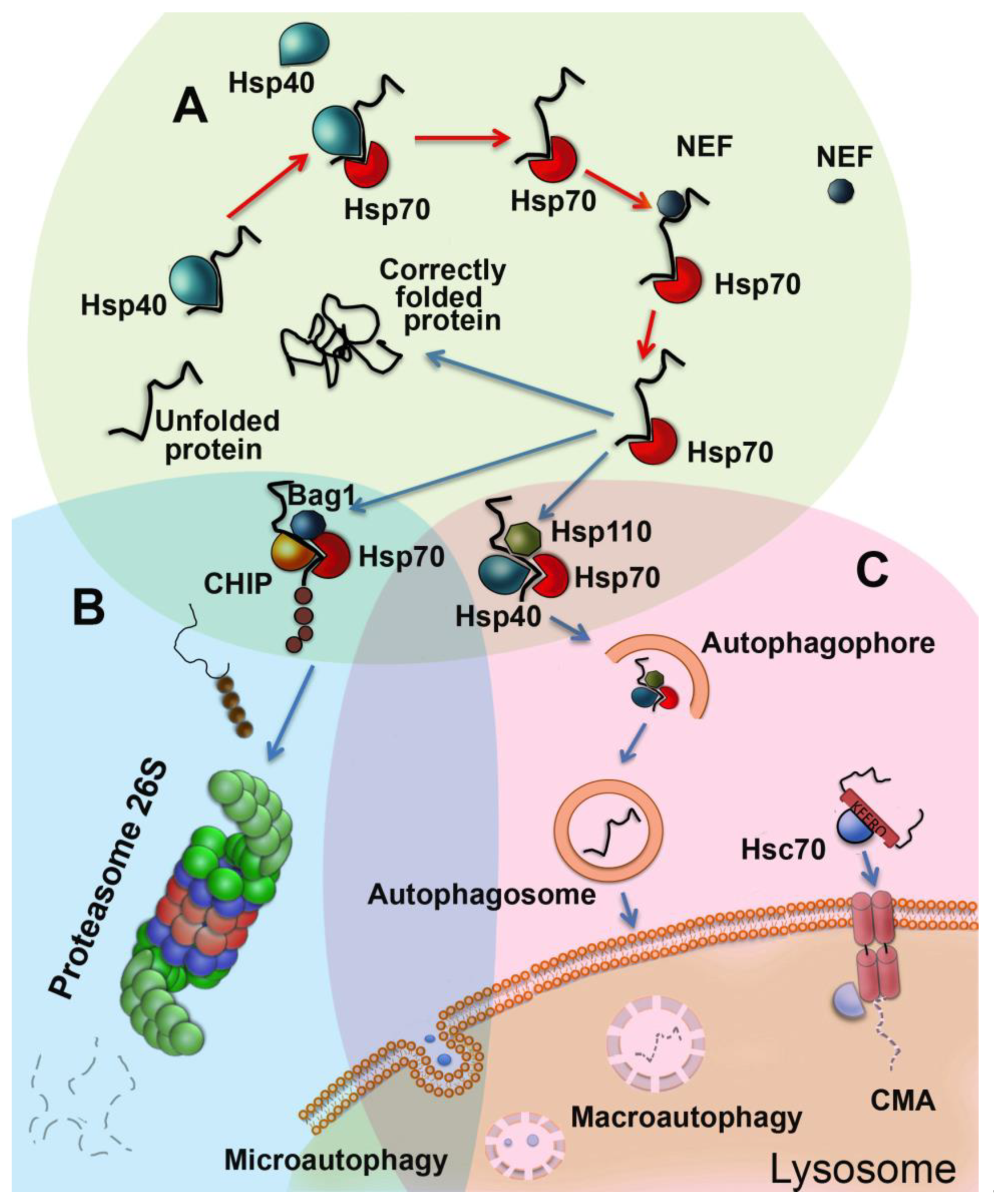
Cells Free Full Text Molecular Chaperones And Proteolytic Machineries Regulate Protein Homeostasis In Aging Cells Html
The Hsp70 family.

. Molecular chaperones to assist the folding of proteins. 6 Slides per page 2 Slides per page EM Packages. None of the above.
Question 6 Molecular chaperones assist proteins in the formation of _______. To get an. The representatives of the Escherichia coli chaperone system GroEL Hsp60.
Chaperones are molecular helpers that assist other proteins with folding. Chaperones of the Hsp70 class and their partner proteins interact with nascent polypeptide chains on ribosomes and prevent their premature misfolding at least until a domain capable of forming a stable structure is. This protein forms structures in mitochondria that.
- Hsp70-type - Chaperonin TriC in eukaryotes and GroELGroES in bacteria What type of protein does not require chaperones at all. Chaperones are molecular helpers that assist other proteins with folding. Correct answer to the question Molecular chaperones assist proteins in the formation of ___.
Molecular chaperones first identified as heat shock proteins Hsps help fold newly synthesized proteins inhibit and reverse the misfolding and aggregation and assist in the degradation of terminally misfolded proteins thereby maintaining cellular proteostasis under physiological and stress conditions Klaips et al 2018. Question 16 Molecular chaperones assist proteins in the formation of 1 structure none of the above amide bonds 3 structure aggregates MacBook. 52 MB 2 Slides per page NB.
It has been shown that members of the heat-shock protein 70 Hsp70 chaperone family assist polyomavirus capsids during infection. Molecular chaperones not only have a function in assisting the folding of newly synthesized proteins they also protect proteins from aggregation and assist in their reconversion to the native state when they have been denatured by high temperatures or other stresses promoting protein unfolding like oxidative stress or heavy metals. A panel of yeast strains with single chaperone gene deletions were used to.
Ntrinsically disordered protein sequences enable High-specificity binding to other proteins. Members of the Hsp70 chaperone family are found in all three surviving domains of life 45At the molecular level Hsp70 is a two-domain protein consisting of a nucleotide-binding domain connected by a long and flexible hydrophobic linker to the substrate-binding domain that can be subdivided into a β-sandwich domain and an α-helical domain. Folding of newly synthesized polypeptides in the crowded cellular environment requires the assistance of so-called molecular chaperone proteins.
One such chaperone is the so-called heat shock protein 60 Hsp60. Molecular chaperones assist proteins in the formation of _______. HSPs are a family of proteins serving as molecular chaperones that prevent the formation of nonspecific.
Physiologically HSPs play a protective role in the homeostasis of the vessel wall but have an impact on immunoinflammatory processes in pathological conditions involved in the. HSPs are a family of proteins serving as molecular chaperones that prevent the formation of nonspecific protein aggregates and assist proteins in the acquisition of their native structures. To provide An understanding of roles structures and mechanisms of molecular chaperone.
31 pages 18 MB Old Packages Finalilities and targets aim. Ranging from multisubunit ring-shaped chaperonin and Hsp100 machines that use their central cavities to bind and compartmentalize action on proteins to machines that use other topologies of recognition binding cellular proteins in an archway or at the surface of a clamp or at the surface of a globular assembly the structures show us the ways and means the cell has. Oct 2006 Chaperone Packed.
In molecular biology molecular chaperones are proteins that assist the conformational folding or unfolding and the assembly or disassembly of other macromolecular structures. Multiple choice 2. One such chaperone is the so-called heat shock protein 60 Hsp60.
However some chaperones are complex oligomeric proteins and one of the intriguing questions is how the chaperones fold. Molecular chaperones catalyze the folding of proteins in the crowded environment of the cell. STOP Make sure you select the FALSE Statement They help prevent the formation of protein aggregates They assist proteins in folding into their correct conformations They specify the three-dimensional shape of proteins They make the protein-folding process in.
None of the above. Chaperones have been widely used to improve the expression of various proteins which are otherwise difficult to produce in E. Molecular chaperones are proteins existing in bacteria and eukaryotic cells which have an important roles to assist cellular homeostasis under normal and detrimental growth situation and cell defences to inhibit aggregation of mis-folded proteins Various genetic sources have molecular chaperones because these agents have critical roles in cell such as.
Molecular chaperones are a special class of heat shock proteins Hsp that assist the folding and formation of the quaternary structure of other proteins both in vivo and in vitro. Small proteins with no large hydrophobic regions. Chaperones are present when the macromolecules perform their normal biological functions and have correctly completed the processes of folding andor assembly.
Show transcribed image text TRUE OR FALSE molecular chaperones help prevent formation of protein aggregates molecular chaperones specify the final three-dimensional shape of proteins. In molecular biology molecular chaperones View the full answer Transcribed image text. Molecular chaperones assist newly synthesized aggregated or misfolded proteins to fold into their native conformations.
In response to stress stimuli mammalian cells activate an ancient signaling pathway leading to the transient expression of heat shock proteins HSPs. 6 Slides per page NB. The chaperones are concerned primarily.
This protein forms structures in mitochondria that. O Question 14 All of the following are roles of molecular chaperones in protein folding EXCEPT. What are the 2 major classes of molecular chaperones.
Describe the structure of GroELGroES chaperonin in E. However the molecular chaperones that assist the formation of recombinant capsid viral protein 1 VP1-derived virus-like particles VLPs in yeast remain unclear.

Protein Folding In The Cytosol A Models For The Chaperone Assisted Download Scientific Diagram
Chaperone Assisted Protein Folding In The E Coli Cytosol Download Scientific Diagram

Molecular Chaperones An Overview Sciencedirect Topics
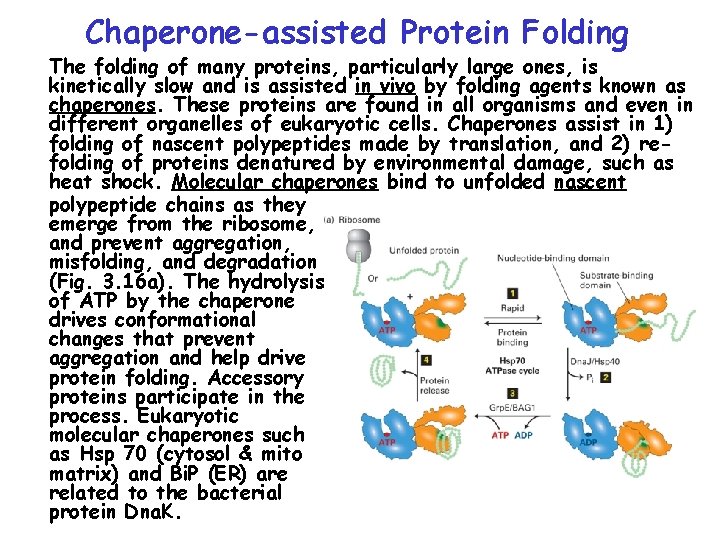
Chap 3 Protein Structure Function Topics Hierarchical Structure

Mechanisms Used By Molecular Chaperones To Maintain Proteins In Their Download Scientific Diagram

Chaperonin Facilitates Protein Folding By Avoiding Polypeptide Collapse Biorxiv

Chaperonin An Overview Sciencedirect Topics
What Is The Role Of Chaperone Proteins In Protein Folding Quora

Chaperone Assisted Folding Of Proteins In Eukaryotes 34 A Early Download Scientific Diagram
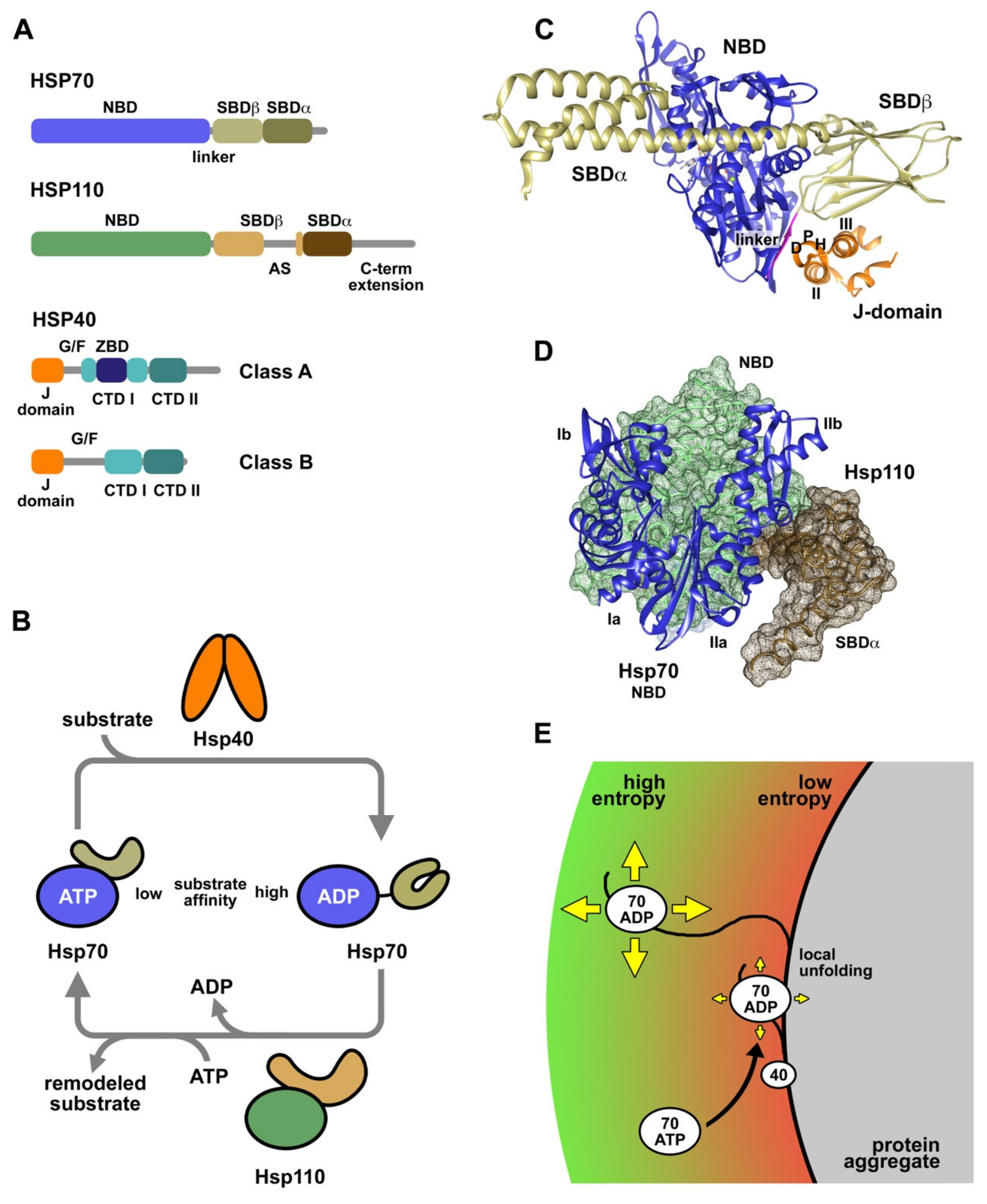
Ijms Free Full Text The Complex Phosphorylation Patterns That Regulate The Activity Of Hsp70 And Its Cochaperones Html

A Model Of Molecular Chaperone Mediated Protein Folding In The Download Scientific Diagram

Chaperone Assisted Folding Of Proteins In Eukaryotes 34 A Early Download Scientific Diagram

The Interactions Of Molecular Chaperones With Client Proteins Why Are They So Weak Journal Of Biological Chemistry

Molecular Chaperones An Overview Sciencedirect Topics
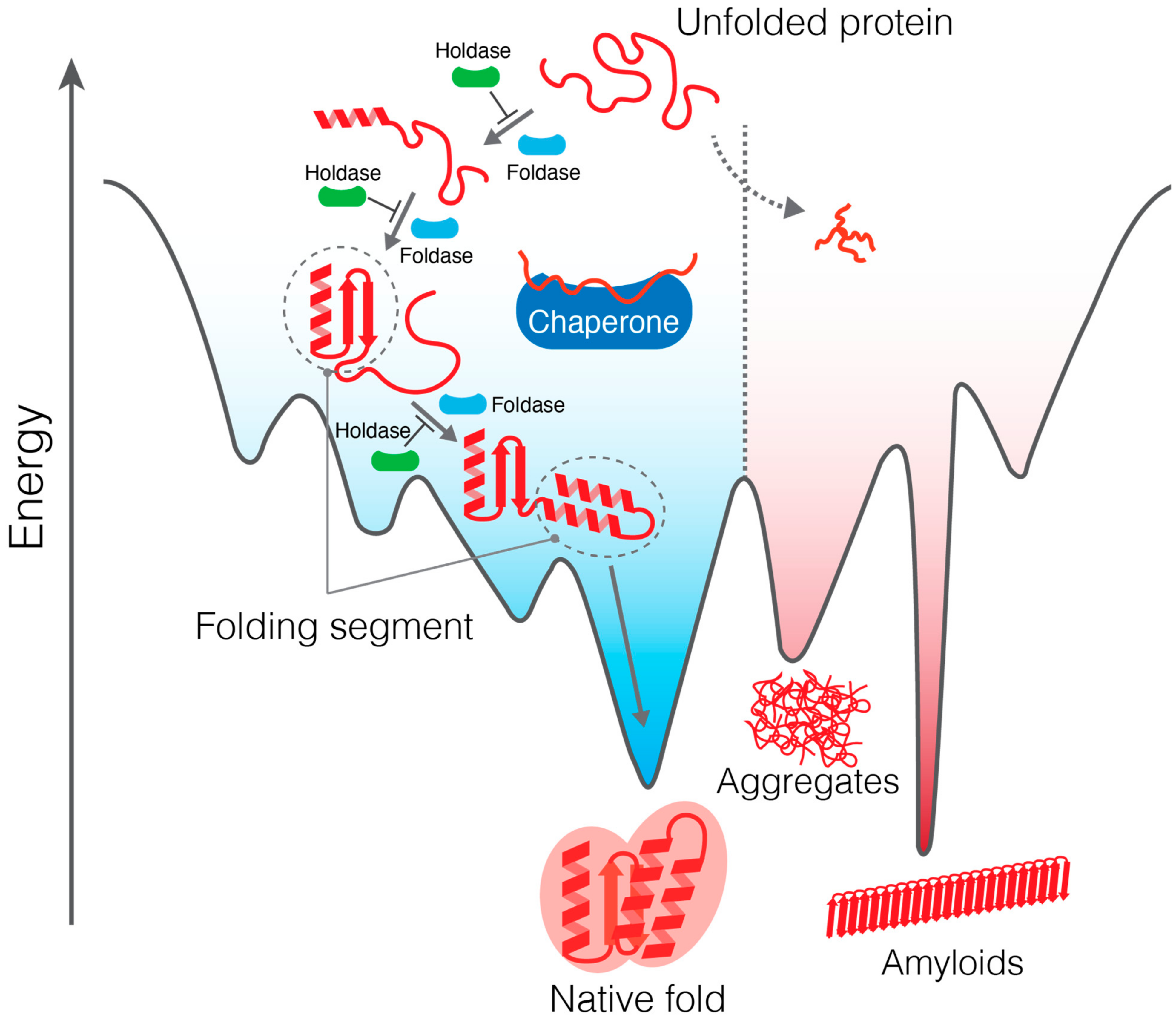
Ijms Free Full Text Structural And Kinetic Views Of Molecular Chaperones In Multidomain Protein Folding Html
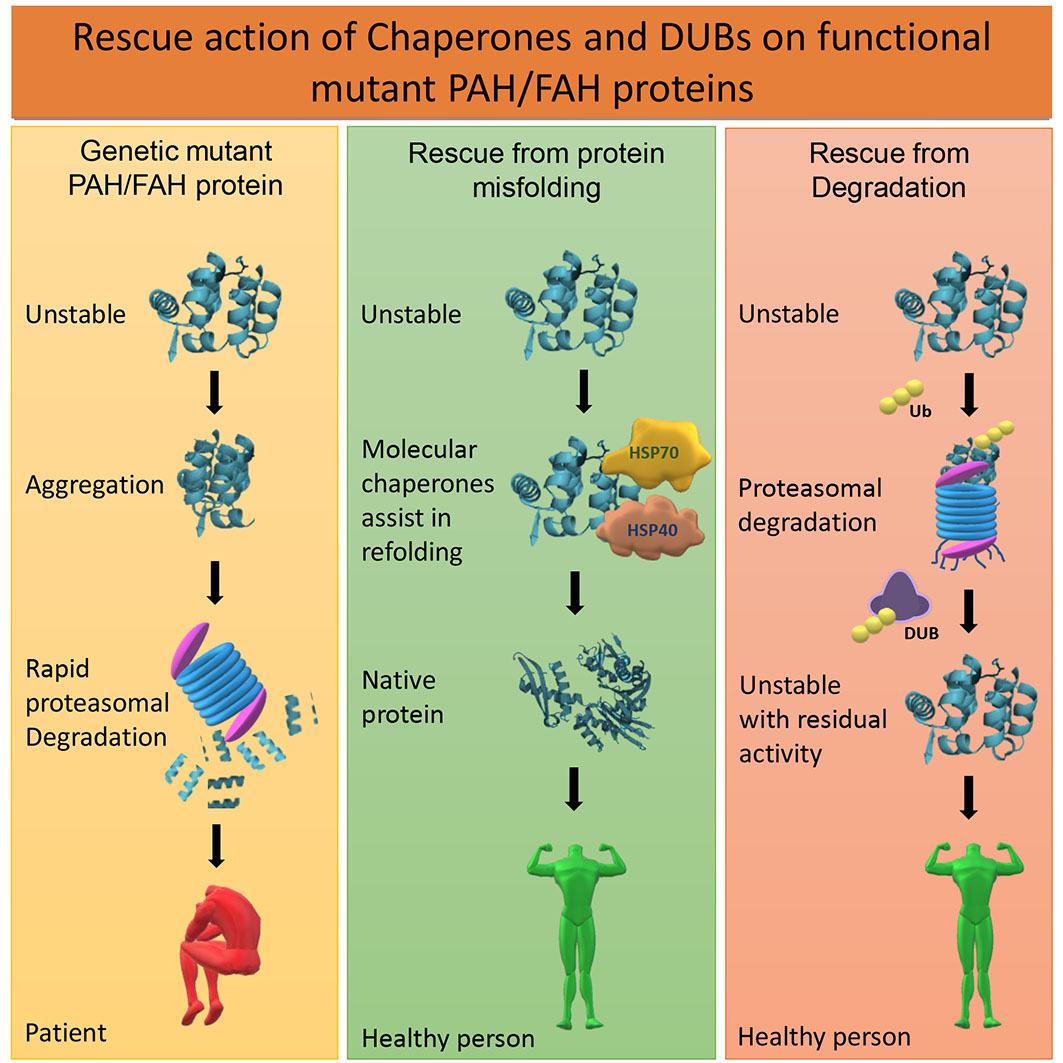
Ijms Free Full Text Protein Degradation And The Pathologic Basis Of Phenylketonuria And Hereditary Tyrosinemia Html
How Molecular Chaperones Dissolve Protein Agg Eurekalert
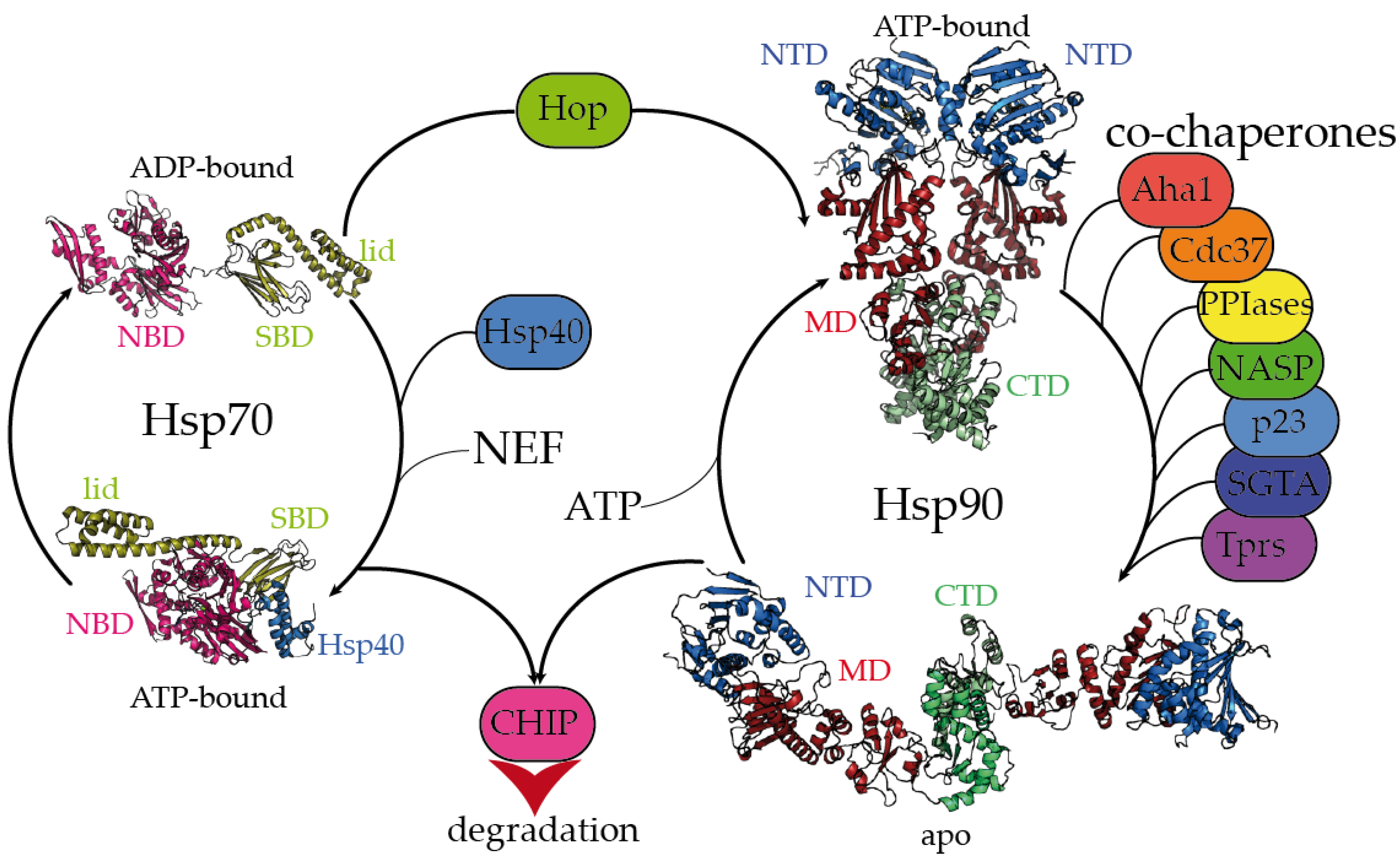
Ijms Free Full Text Mechanistic Insights Into The Role Of Molecular Chaperones In Protein Misfolding Diseases From Molecular Recognition To Amyloid Disassembly Html
Comments
Post a Comment